Epidemic of Autoimmune Diseases Blamed on Shift in Microbiome
The Sum of Our Parts by Janice Dietert and Rodney Dietert
The Scientist, Exploring Life, Inspiring Innovation July 1, 2015
In less than 100 years, leading diseases and causes of death have shifted dramatically away from infectious diseases and heavily toward noncommunicable diseases (NCDs), not just in developed countries, but around the globe. NCDs are now the number one killer worldwide, accounting for 63 percent of all mortalities.1 There is no question that environmental variables, including exposure to cigarette smoke, certain dietary factors, and chemicals such as heavy metals, pesticides, endocrine disruptors, or particular drugs, increase one’s risk of developing an NCD. Psychosocial stressors also play a role. But any assumption that the ongoing NCD epidemic is due solely to external factors would be missing a key part of the story: the human microbiome. In reality, the NCD epidemic is as much about the ways we have altered our microbiomes in recent decades as it is about our changing external environment.
 Our microbial gatekeeper
Our microbial gatekeeper
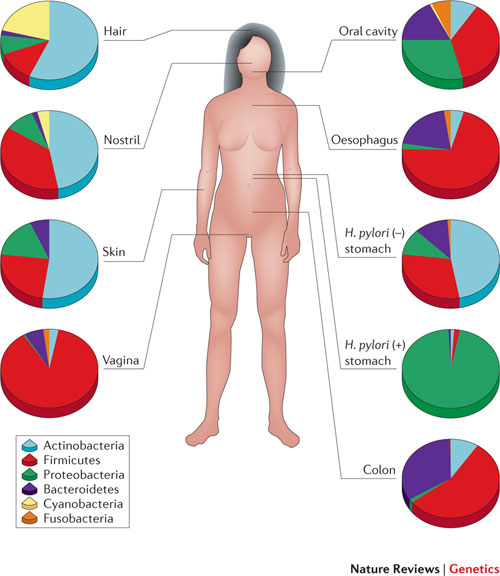
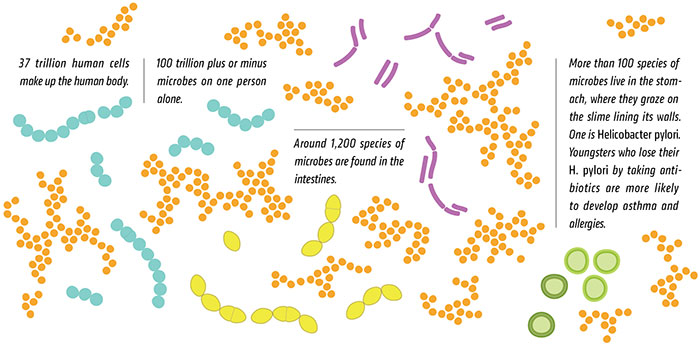
The human microbiome plays a critical role as a filter between us and the world. In fact, it is the microbiome that determines our actual exposure to the environment. Substances such as foods, drugs, and environmental chemicals—collectively termed xenobiotics—must first pass through the layers of microbiota on the skin, in the gut, and in the airways where, depending upon the microbes present, the chemicals will be sequestered, excluded, or metabolized before they ever enter our cells. The common gut actinobacteriumEggerthella lenta, for example, can significantly change the potency of the cardiac drug digoxin.2Likewise, microbiome composition affects the toxicity of certain environmental chemicals such as arsenic, with some sulfur-reducing gut bacteria able to generate highly toxic, thiolated species of arsenic, thereby increasing health risks following exposure.3 And, of course, diverse gut microbes are critical components of our gastrointestinal system, helping us process the otherwise hard-to-digest foods we eat.
There is also a flip side to the xenobiotic-microbiome relationship: the external environment affects the composition of our microbial populations. Even some xenobiotics that were previously thought to be safe may need to be reexamined in light of effects on the microbiome. For example, commonly used food emulsifiers such as polysorbate 80 and carboxymethylcellulose have been reported to adversely affect the microbiome of rodents, predisposing them to chronic inflammation and elevated risk of metabolic syndrome. In one study, mice that drank the emulsifiers in water showed reduced overall diversity of the gut microbiota, decreased representation of generally beneficial Bacteroidales species, and higher numbers of some potentially pathogenic bacteria, such as Ruminococcus gnavus.4 In some rodent strains, exposure to the emulsifiers also thinned the mucus barrier, reducing the physical distance between bacteria residing on the surface of the barrier and gut epithelial cells by more than 50 percent. Such alterations can affect the interactions between bacteria and cells of the innate immune system, increasing the risk of inflammation-driven disease. Not coincidentally, microbiomes that have been impoverished or unbalanced by environmental factors often have a skewed bacterial metabolism, affecting their host’s energy utilization, hormone status, and control of inflammation.
Thus, it should be no surprise that altered microbiomes and elevated risk of NCDs go hand in hand. Myriad studies have linked specific NCDs to an altered diversity of gut microbiota in early life, with possible risk factors including maternal and infant diet, birth delivery mode, perinatal environmental toxicant exposures, and psychosocial stressors.5,6 Many disease-associated microbiomes can serve as a type of fingerprint, reflecting the underlying disease condition. In some cases, these skewed, limited-diversity microbial communities may help cause or promote the disease; in others, they may be a consequence.
Self-completion
Given the undeniable importance of commensal microbes in both training our immune systems and serving as a barrier between ourselves and the outside world, one of us (R.D.) has posited that a complete microbiome, seeded at birth, is absolutely critical for a healthful life, an idea called “the completed self hypothesis.”13 Single-celled organisms from all three domains of life—eukaryotes, archaea, and bacteria—join our mammalian cells to create a superorganism. Inadequate or inappropriate seeding of the microbiome is in many ways the equivalent of being born with a serious birth defect, resulting in inappropriately matured physiological systems.14 In the absence of effective microbiome-based training, the immune system does not learn what is safe outside of the body, resulting in haphazard, inappropriate reactions to innocuous environmental factors—allergens such as pollen, mold, cat dander, and peanuts. It also fails to properly recognize and ignore internal targets, resulting in autoimmune and inflammatory responses that are misdirected, ineffective, and sometimes never-ending. Such reactions can eventually compromise the function of our own tissues and organs.
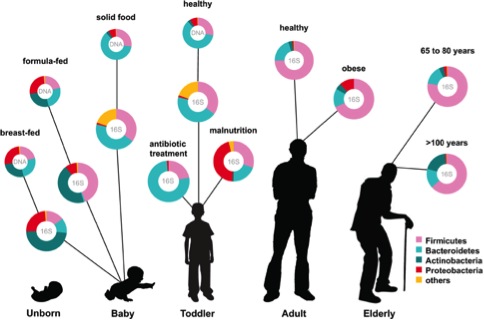
A newborn’s microbiome is largely inherited from the mother, with birth being the most pivotal step in seeding. During vaginal delivery, the passage of the baby down the birth canal allows exposure not only to the vaginal microbiota but also to a film of maternal intestinal flora. This process is thought to provide direct seeding of the newborn’s gut with maternal microbes. Skin-to-skin seeding is also important at birth. When natural childbirth is interrupted—for example, by cesarean delivery—the baby is seeded by default with microbes from the local environment, typically from the largely sterile hospital staff and equipment. Invariably, this results in incomplete and/or inappropriate infant microbial seeding. Indeed, numerous studies have suggested that cesarean-delivered babies typically have altered immune profiles and are at an elevated risk for NCDs such as asthma, type 1 diabetes, and obesity. A recent study of 98 Swedish infants and their mothers, for example, found that cesarean delivery significantly blocked vertical transmission of the maternal microbiome to the infant.15 Additionally, the microbiome transition toward an adult-type profile was shaped by the infant’s feeding pattern after birth, including both breast-feeding and the transition to solid foods.
Disruptions to complete microbiome transfer can also occur before birth, as the mother’s microbial makeup is influenced by her diet, environmental exposures, and health. Microbiota originating from a mother afflicted with one or more NCDs or from a mother who was treated with antibiotics during pregnancy are likely to differ from the microbiota transferred from a mother who is NCD- and antibiotic-free.
In contrast to our human genome, our microbial genome is more amenable to adjustment by altering the composition of the microbial communities inhabiting our bodies. Some researchers and doctors have already recognized the power of microbiome manipulation—think probiotics and fecal transplants (Microb Ecol Health Dis, 26:25877, 2015). Probiotic mixtures can be ingested to shift microbial balance and metabolism in the gut, translating to potentially useful physiological alterations. Recent reports suggest that probiotics can prevent diarrhea in children taking antibiotics, for example, as well as increase the efficacy and reduce the side effects of anti–Helicobacter pylori therapies and aid peanut oral immunotherapy for the treatment of peanut allergy. The more radical approach of fecal transplantation, in which microbiota are installed in the gut via a gastric or nasoduodenal tube, an enema, or colonoscope, or orally administered frozen capsules, has proven successful for the treatment of Clostridium difficile infection (Infect Dis Clin North Am, 29:109-22, 2015), and other potential uses are currently under investigation. Fecal transplants have also been used subsequent to antibiotic administration to reinstate a healthy microbiome. Identification and selection of donor microbes is likely to be an important future consideration for these therapies.
While microbiome manipulation may have benefits at any age, once certain developmental programming of our physiological systems has occurred, it is likely to be much more difficult to correct underlying dysfunctions. Intervention early in life is the most comprehensive technique, as it allows for self-completion in the newborn prior to most postnatal developmental programming. We believe that no baby should go unseeded or be left to haphazardly acquire the daily menu of microbes from a given hospital environment. If elective cesarean delivery is planned, deliberate seeding of the baby should be considered. Maria Gloria Dominguez-Bello of the New York University Langone Medical Center, for example, has promoted the use of vaginal swabs immediately after cesarean birth to simulate the baby’s exposure to maternal microbes in the birth canal, and preliminary results are encouraging (Trends Mol Med, 21:109-17, 2015). Potential medical complications should be considered in any decision regarding microbial manipulation therapies. These must be balanced against the immune and other long-term health risks that are created if the baby cannot self-complete as a superorganism.
https://www.the-scientist.com/?articles.view/articleNo/43379/title/The-Sum-of-Our-Parts