Managing Refractory (Non-Resolving) Celiac Disease
 The diagnosis and management of refractory celiac disease remains challenging, but ongoing studies can provide the proper diagnostic criteria and identify the optimal management strategies, according to a new American Gastroenterological Association expert review published in Gastroenterology.
The diagnosis and management of refractory celiac disease remains challenging, but ongoing studies can provide the proper diagnostic criteria and identify the optimal management strategies, according to a new American Gastroenterological Association expert review published in Gastroenterology.
- Carolyn Crist, mdedge.com/gihepnews 1
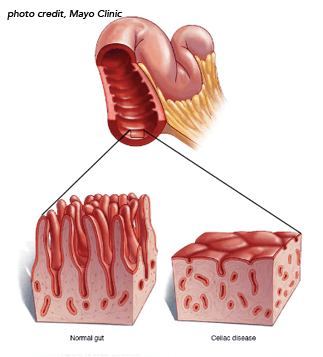
Celiac disease is present in about 1% of the U.S. population and can cause various symptoms, wrote Peter H. R. Green, MD, director of the Celiac Disease Center at Columbia University, New York, and colleagues. Adhering to a strict gluten-free diet can improve symptoms, normalize serum antibody levels, and reverse small bowel villous atrophy. However, persistent or recurrent symptoms and elevated celiac antibodies can persist in some patients after a year of trying a gluten-free diet, a condition called non-responsive celiac disease. In some patients, this raises concern for refractory celiac disease, or RCD.
“RCD is believed to occur in only approximately 1% of patients with celiac disease, although this may be an overestimate, as data are obtained from referral centers,” the authors wrote.
RCD can be classified into two subtypes with different diagnostic criteria, prognoses, and therapy responses. The first, called RCD1, is characterized by villous atrophy but has intraepithelial lymphocytes similar to conventional celiac disease. The other, called RCD2, is characterized by aberrant clonal T-cell expansion in the intestinal tract and other organs, has a poorer prognosis than RCD1, and has a risk of developing ulcerative jejuno-ileitis or enteropathy-associated T-cell lymphoma.
The experts developed 10 clinical practice advice statements based on a review of the published literature and expert opinion.
1. First, in patients who have persistent or recurring symptoms, an initial celiac disease diagnosis should be confirmed through review of prior diagnostic testing, including serologies, endoscopies, and histologic findings. Celiac disease can overlap with other gastrointestinal conditions, and some pathologic findings aren’t specific to celiac disease. Results of serologic testing with tissue transglutaminase immunoglobulin A, de-amidated gliadin peptide IgA and IgG, and endomysial antibodies should be reviewed or obtained if not previously performed.
2. Next, in those with confirmed but non-responsive celiac disease, ongoing gluten ingestion should be excluded as a cause of symptoms with serologic testing, dietitian review, and potentially detection of immunogenic peptides in stool or urine samples. The authors noted that persistent gluten ingestion, whether intentional or inadvertent, accounts for 40%-50% of patients with non-responsive celiac disease. In these cases, esophago-gastroduodenoscopy and small bowel biopsies should be performed to look for persistent villous atrophy, which can also be caused by common variable immunodeficiency, autoimmune enteropathy, tropical sprue, and medication-induced enteropathy. Patients with villous atrophy due to other causes won’t respond to a gluten-free diet.
3. After excluding gluten, clinicians should perform a systematic evaluation for other potential causes of symptoms, including functional bowel disorders, lactose or fructose intolerance, microscopic colitis, pancreatic insufficiency, inflammatory bowel disease, and small intestinal bacterial growth. Irritable bowel syndrome, for instance, may contribute to persistent symptoms and respond to fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) restriction. RCD should be strongly considered in patients with persistent symptoms or signs of malabsorption after the exclusion of the other causes.
4. To distinguish between the two subtypes of RCD and exclude enteropathy-associated T-cell lymphoma, clinicians should use flow cytometry, immuno-histochemistry, and T-cell receptor rearrangement studies. RCD1 has a normal intraepithelial lymphocyte population, and RCD2 has an aberrant, clonal intraepithelial lymphocyte population. Consulting with a hemato-pathologist may be necessary to interpret these studies.
5. After RCD2 is diagnosed, complications such as enteropathy-associated T-cell lymphoma and ulcerative jejuno-ileitis should be excluded through small bowel imaging with capsule endoscopy and either computed tomography (CT) or magnetic resonance enterography. In general, the extent and severity of villous atrophy is greater in patients with RCD2, compared with RCD1.
6. In patients diagnosed with RCD, clinicians should complete a detailed nutritional assessment with investigation of micronutrient and macronutrient deficiencies. Check albumin as an independent prognostic factor. Then, try to correct deficiencies with oral supplements. Malnourished patients may need enteral support, and those with severe malnutrition due to malabsorption may need parenteral support.
7. So far, RCD management suggestions are based on small retrospective studies and expert opinion, with minimal prospective data and no Food and Drug Administration–approved therapies. The goals should be to improve symptoms and duodenal mucosal abnormalities, manage malnutrition, and prevent lymphoma. Glucocorticoids are considered first-line therapy, typically open-capsule budesonide given as 3 mg three times daily. Prednisone serves as an alternative with proven efficacy but a higher risk for adverse effects.
8. The optimal choice for second-line therapy is unknown, but the addition of an immunosuppressant agent to steroids appears to be effective in RCD1, including azathioprine, mercaptopurine, and tioguanine. The best treatment for RCD2 is unknown, though clinical response has been reported with steroids, and cladribine has been well tolerated in some patients.
9. Patients with RCD who don’t respond to steroids may benefit from referral to a center with expertise for management or evaluation for inclusion in clinical trials. Frequent medical visits are advised until the disease is well controlled, with regular follow-up after that.
10. Ultimately, “patients with RCD benefit from evaluation and regular follow-up by a multidisciplinary team, including gastroenterologists and dietitians, to assess clinical and histologic response to therapy,” the authors wrote. “Identify local experts with expertise in celiac disease to assist with management.”













