Ten Things You Hope Your Gastroenterologist Knows About Celiac Disease
“How CD is diagnosed has changed over time, and confusion abounds regarding the use and interpretation of diagnostic tests, which are often confounded by adoption of the gluten-free diet (GFD). Herein, we address 10 important things that gastroenterologists need to know about CD, which are based on current evidence and our experience in Mayo Clinic’s Celiac Disease Clinic, starting with the best blood test(s) to detect it,” by Amy S. Oxentenko and Joseph A. Murray, Clinical Gastroenterology Hepatology Journal (2015;13(8):1396-1404), as published in Medscape
(1) The immunoglobulin A tissue transglutaminase is the single best serologic test to use for the detection of CD.
Serologic testing for CD has significantly advanced during the past 2 decades. The long-used anti-gliadin antibodies have been supplanted by serology with better test characteristics. For example, endomysial antibody (EMA), used for more than 20 years, has specificity of 99%, although the sensitivity varies because of the technical issues inherent in direct immunofluorescence. This high specificity keeps EMA in use despite tissue transglutaminase (TTG) being identified as the targeted epitope. EMA is used primarily when discordance exists between other markers and histologic findings or when TTG immunoglobulin (Ig) A antibodies are equivocal.
TTG antibodies come in both IgA-based and IgG-based assays, which are performed with enzyme-linked immunosorbent assay by using human recombinant/derived proteins. IgA TTG has high sensitivity and specificity of ~98% and is the endorsed serologic marker for evaluating CD. It is well-known that IgA deficiency affects 2%–3% of CD patients and occurs in 1:131 patients tested for CD;[4] thus, IgA-based assays alone are not always reliable. To avoid missing IgA-deficient CD, a serologic cascade testing starting with serum IgA level can be performed, and if normal, an IgA TTG is adequate; however, if the IgA level is low or absent, IgG-based testing with deamidated gliadin peptide (DGP) and/or TTG could be added/substituted. The accuracy of IgG TTG is poor (30%–70%) in IgA sufficiency, so this test in isolation should not be used for routine CD screening.However, IgG TTG performs well with known IgA deficiency, with sensitivity and specificity approaching 95%.
Point-of-care finger stick TTG antibody testing has been developed as a rapid screen for CD, and although the specificity was reportedly 100%, the sensitivity was only 82%. It cannot be recommended until sensitivity improves.
The newest serologic marker is the DGP antibody, which comes in both IgA-based and IgG-based assays and is significantly better than anti-gliadin antibody testing. Despite specificity that is close to TTG assays, the sensitivity of either the IgA-based or IgG-based DGP in isolation is lower; however, a combined IgA/IgG DGP panel has accuracy equivalent to IgA-based TTG.
In children older than 2 years, IgA TTG is the preferred test. Serologic markers may have decreased sensitivity in children younger than 2 years. The combination of DGP IgA/IgG with IgA TTG is the recommended strategy.
Should panels of serologic studies for CD be more widely used? The answer is no. The widespread use of panels would not be cost-effective as first-line testing; although it may slightly improve overall sensitivity, it reduces specificity, leading to unnecessary endoscopy.
Practical Suggestion on Serological Testing:
IgA-based TTG is the serologic test of choice for evaluating CD in patients consuming a gluten-containing diet. IgG-based tests are needed in IgA deficiency. The use of celiac cascade testing starting with serum IgA level could direct downstream serology. Equivocal or discrepant serologic tests should be interpreted with caution. Details …
Nine More Things Your Gastroenterologist Should Know:
(2) CD can be recognized endoscopically, and water immersion enhances villi detection, although a normal endoscopic appearance does not preclude the diagnosis. Details …
(3) It is recommended that 4 biopsies be taken from the second part of the duodenum and 2 bulb biopsies be taken at the 9 o’clock and 12 o’clock positions to maximize the sensitivity for histologic confirmation of CD. Details …
(4) Consider serologic testing of first-degree relatives, patients with type 1 diabetes mellitus, Down’s, Turner’s, and Williams’ syndromes, as well as those with premature osteoporosis, iron deficiency, abnormal liver biochemistries, and other manifestations of CD. Details …
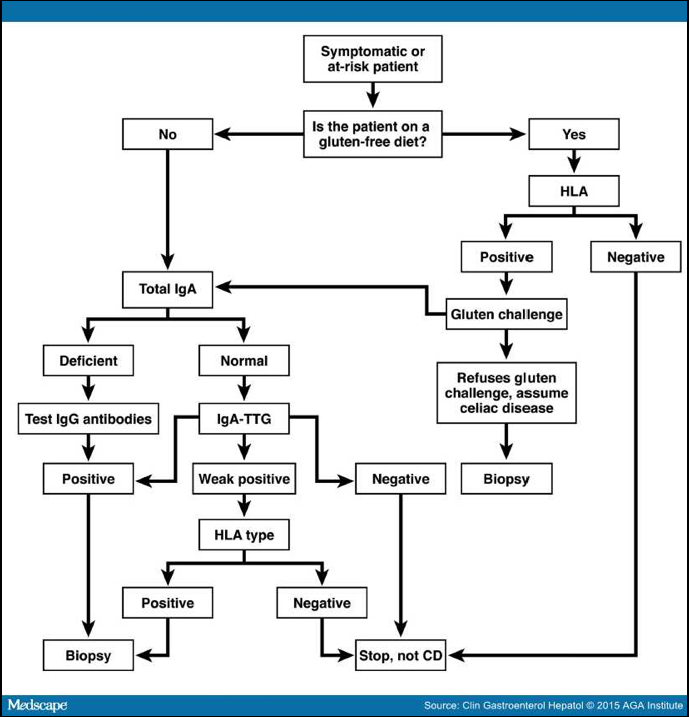
(5) Patients already on a prolonged gluten-free diet (GFD) should be tested for the presence of HLA DQ2 or DQ8, thereby avoiding the need for further evaluation of CD in non-allelic carriers. Details …
(6) The basic treatment of CD is a strict, lifelong GFD, enabled by an expert dietitian. Details …
(7) Newly diagnosed adults with CD should be assessed for micronutrient deficiencies (iron, B12, folate, zinc, copper), fat soluble vitamin deficiencies (vitamin D), and bone densitometry. Details …
(8) All patients diagnosed with CD should have clinical follow-up to ensure response and adherence to a GFD. Details …
(9) In those with persistent or relapsing symptoms, the robustness of the original diagnosis should be reviewed, gluten exposure sought, and a systematic evaluation for alternative and associated diseases performed. Details …
(10) Evaluate those with refractory disease for malignant transformation. Details …